Key Applications
- There are several analytical techniques that manufacturers can use to determine sulphate in TiO2. One commonly used method is ion chromatography (IC), which involves separating sulphate ions from other anions in the sample using a chromatographic column and detecting them with a conductivity detector. This method is highly sensitive and can accurately quantify sulphate levels down to very low concentrations.
Overall, buff percentage is a critical factor that manufacturers of titanium dioxide must carefully manage to ensure the quality, consistency, and cost-effectiveness of their products. By investing in advanced technology and processes to control buff percentage, manufacturers can meet the specific requirements of their customers and maintain a competitive edge in the market. As the demand for titanium dioxide continues to grow across various industries, manufacturers must continue to innovate and improve their processes to meet the evolving needs of their customers.
- China is one of the largest producers of titanium oxide in the world, and the industry plays a significant role in the country's economy. Titanium oxide, also known as titanium dioxide, is a naturally occurring oxide of titanium with the chemical formula TiO2. It is commonly used as a pigment in a wide range of products, including paints, coatings, plastics, and cosmetics.
1345-05-7
lithopone supplier 30% complies with both the REACH and Indirect Food Regulations, as well as with many European regulations regarding Toys, Packaging, Resins, etc…
Neurotoxicity

Following a request for assessment in 2020 by the EU, the European Food Safety Authority (EFSA) assessed E171, particularly for its genotoxicity. In 2022, the agency deemed the food additive no longer safe for use.
Different dermal cell types have been reported to differ in their sensitivity to nano-sized TiO2 . Kiss et al. exposed human keratinocytes (HaCaT), human dermal fibroblast cells, sebaceous gland cells (SZ95) and primary human melanocytes to 9 nm-sized TiO2 particles at concentrations from 0.15 to 15 μg/cm2 for up to 4 days. The particles were detected in the cytoplasm and perinuclear region in fibroblasts and melanocytes, but not in kerati-nocytes or sebaceous cells. The uptake was associated with an increase in the intracellular Ca2+ concentration. A dose- and time-dependent decrease in cell proliferation was evident in all cell types, whereas in fibroblasts an increase in cell death via apoptosis has also been observed. Anatase TiO2 in 20–100 nm-sized form has been shown to be cytotoxic in mouse L929 fibroblasts. The decrease in cell viability was associated with an increase in the production of ROS and the depletion of glutathione. The particles were internalized and detected within lysosomes. In human keratinocytes exposed for 24 h to non-illuminated, 7 nm-sized anatase TiO2, a cluster analysis of the gene expression revealed that genes involved in the “inflammatory response” and “cell adhesion”, but not those involved in “oxidative stress” and “apoptosis”, were up-regulated. The results suggest that non-illuminated TiO2 particles have no significant impact on ROS-associated oxidative damage, but affect the cell-matrix adhesion in keratinocytes in extracellular matrix remodelling. In human keratinocytes, Kocbek et al. investigated the adverse effects of 25 nm-sized anatase TiO2 (5 and 10 μg/ml) after 3 months of exposure and found no changes in the cell growth and morphology, mitochondrial function and cell cycle distribution. The only change was a larger number of nanotubular intracellular connections in TiO2-exposed cells compared to non-exposed cells. Although the authors proposed that this change may indicate a cellular transformation, the significance of this finding is not clear. On the other hand, Dunford et al. studied the genotoxicity of UV-irradiated TiO2 extracted from sunscreen lotions, and reported severe damage to plasmid and nuclear DNA in human fibroblasts. Manitol (antioxidant) prevented DNA damage, implying that the genotoxicity was mediated by ROS.
Applications
 Elementis
Elementis It can split water molecules or decompose organic compounds when exposed to light, which is a promising feature for environmental clean-up operations and renewable energy initiatives It can split water molecules or decompose organic compounds when exposed to light, which is a promising feature for environmental clean-up operations and renewable energy initiatives
It can split water molecules or decompose organic compounds when exposed to light, which is a promising feature for environmental clean-up operations and renewable energy initiatives It can split water molecules or decompose organic compounds when exposed to light, which is a promising feature for environmental clean-up operations and renewable energy initiatives r 5566 titanium dioxide. Furthermore, when titanium dioxide nanoparticles are incorporated into cement or concrete, they can endow self-cleaning properties to architectural surfaces by promoting the breakdown of pollutants like nitrogen oxides under UV light.
r 5566 titanium dioxide. Furthermore, when titanium dioxide nanoparticles are incorporated into cement or concrete, they can endow self-cleaning properties to architectural surfaces by promoting the breakdown of pollutants like nitrogen oxides under UV light.Tio2 Powder CR-930 Titanium Dioxide Free Sample
Animal studies show exposure to titanium dioxide is linked to immunotoxicity, inflammation and neurotoxicity.
While the FDA maintains that the regulated use of titanium dioxide is safe, the European Food Safety Authority and some other experts warn of potential, serious health risks.
Residue of mash (wm)
14 Max
Fig. 7. Lipid peroxidation measured on samples of MSSA with: A) 0.2 mg/mL P25TiO2NPs; B) 0.02 mg/mL P25TiO2NPs; C) 0.2 mg/mL VitaminB2@P25TiO2NPs; D) VitaminB2@P25TiO2NPs 0.02 mg/mL after 3 h of irradiation (red) and 6 h (blue). MDA could not be detected after 6 h of irradiation in a sample with P25TiO2NPs. Error bars are too small to be seen in graphic and p < 0.05 between C-D and A-B.
Specific gravity:
In industrial settings, people can be exposed to titanium dioxide through inhalation. Inhalation exposure to titanium dioxide is exceedingly rare for most people.
Titanium dioxide can amplify and brighten white opacity because of its exceptional light-scattering properties. In food and drugs, these properties help to define colors clearly and can prevent products from UV degradation.
Zinc oxide. Zinc oxide is a popular cross-linking agent for rubber and for various resins. It is essential in the formulation of solvent-borne polychloroprene adhesives. Furthermore, zinc oxide is a good UV stabilizer, has biocidal activity and has a relatively high refractive index (2.0) which makes it an efficient white pigment. Some typical properties are: density 5.6 g/cm3; particle size 0.036-3 μm; oil absorption 10–20 g/100 g; specific surface area 10–45 m2/g. Zinc oxide is produced by reaction of the metal in the vapour state with oxygen. Zinc oxide is nonporous and is quite pure. Thus, the high surface area of some grades is due to the small particle size of zinc oxide. Some grades, especially for use in the rubber industry, are surface modified by deposition of 0.2-0.4% of stearic acid, propionic acid, or light oil [47].
Résumé–Cet article traite de la découverte de lithopone phosphorescent sur des dessins à l'aquarelle, datés entre 1890 et 1905, de l'artiste Américain John La Farge et de l'histoire du lithopone dans l'industrie des pigments à la fin du 19e et au début du 20e siècle. Malgré de nombreuses qualités souhaitables pour une utilisation en tant que blanc dans les aquarelles et les peintures à l'huile, le développement du lithopone comme pigment pour artistes a été compliqué de par sa tendance à noircir lorsqu'il est exposé au soleil. Sa disponibilité et son usage par les artistes demeurent incertains parce que les catalogues des marchands de couleurs n'étaient généralement pas explicites à indiquer si les pigments blancs contenaient du lithopone. De plus, lors d'un examen visuel, le lithopone peut être confondu avec le blanc de plomb et sa phosphorescence de courte durée peut facilement être ignorée par l'observateur non averti. À ce jour, le lithopone phosphorescent a seulement été documenté sur une autre œuvre: une aquarelle de Van Gogh. En plus de l'histoire de la fabrication du lithopone, cet article décrit le mécanisme de sa phosphorescence et son identification à l'aide de la spectroscopie Raman et de la spectrofluorimétrie.
Adjustment of Tariff Rates in 2017
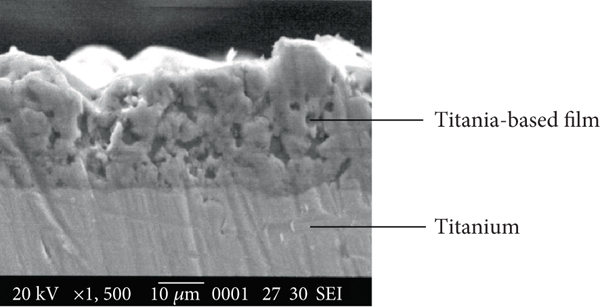 inner wall coating factories. Many factories are now producing coatings that are low in volatile organic compounds (VOCs) and free from harmful chemicals. These environmentally friendly coatings not only benefit the health of occupants but also contribute to a more sustainable future.
inner wall coating factories. Many factories are now producing coatings that are low in volatile organic compounds (VOCs) and free from harmful chemicals. These environmentally friendly coatings not only benefit the health of occupants but also contribute to a more sustainable future.